Chlorine is often removed from a process and there are a number of potential reasons why. Chlorine might damage an RO membrane or turn food brown in an unsightly manner. Chlorine removal presents several challenges to instrumentation manufacturers and engineers, but did you know that…
…ORP can effectively control sodium bisulphite dosing to remove chlorine?
…ORP can limit the dose of excess sodium bisulphite which can lead to problems later in the process?
…ORP is very sensitive to changes in chlorine at very low levels?
How do you effectively control chlorine removal?
Logically, it would be perfectly reasonable to take a standard chlorine probe and dose a reducing agent such as sodium bisulphite until the probe gets to zero. Then if the probe does increase from zero, dose more reducing agent.
Unfortunately due to the inherent electrochemical properties in chlorine sensors, this perfectly logical solution won’t work. This is because amperometric chlorine probes require the presence of some chlorine in order to function properly. This is generally called polarization and the longer a probe has been in water without chlorine, the longer it will take to see chlorine when it is reintroduced. This isn’t due to manufacturing quality or to design flaws. This is a fundamental electrochemical property that is hard to engineer out.
Some amperometric probes are more resistant to this effect than others. They are suitable for applications where chlorine may intermittently reach zero, or where the probe can be periodically reset in chlorinated water (typically potable water). Pi’s HaloSense Zero has been built with this in mind. This amperometric zero chlorine sensor is being successfully used in RO membrane applications and many other low-level chlorine processes.
However, in applications where reducing agents are used to remove chlorine, a more elegant solution is to use ORP. An ORP sensor measures oxidation reduction potential.

The reaction between chlorine and a reducing agent is a reduction-oxidation (redox) reaction. This means that ORP can be used to control this process and can ensure the removal of chlorine, whilst simultaneously preventing the overdosing of a reducing agent.
Below is a simplified example of the reaction between HOCl (free chlorine) and NaHSO3 (sodium bisulphite).
- 2NAHSO3 + 2HOCl → H2SO4 + 2HCl + Na2SO4
When free chlorine is in excess, the ORP is raised and when sodium bisulphite is in excess, the ORP is lowered.
At low levels, a very small change in the concentration of chlorine will result in a large change in ORP. This is because the relationship between ORP and the concentrations of oxidants and reducing agents is logarithmic. The same also goes for the concentration of reducing agent (such as bisulphite), once an excess has been reached.
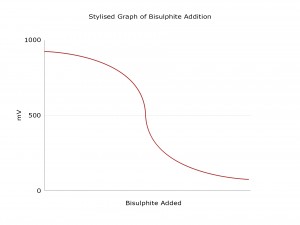 The ORPSense from Pi can be used to control chlorine removal. An ORP titration curve is necessary to choose a setpoint for ORP. The graph shows a typical ORP titration curve, though the exact values will be different for each process. It is important to test each new installation and to adjust the ORP setpoint accordingly. Once this titration curve has been determined, there is no continual calibration involved with ORP sensors.
The ORPSense from Pi can be used to control chlorine removal. An ORP titration curve is necessary to choose a setpoint for ORP. The graph shows a typical ORP titration curve, though the exact values will be different for each process. It is important to test each new installation and to adjust the ORP setpoint accordingly. Once this titration curve has been determined, there is no continual calibration involved with ORP sensors.
It is worth acknowledging that ORP can be affected by many other variables in the process water, such as conductivity, pH or temperature, and that this method of control won’t be suitable for all processes. Combined with Pi’s CRONOS® or CRIUS® controller, Pi’s ORPSense can be installed alongside other sensors like the zero chlorine sensor, pH or conductivity sensors to help give you a more complete picture of your process.
If you need to control a process where chlorine is removed with chemicals, a carbon filter or even with UV, Pi can help you by providing world class instrumentation and application expertise.
NB. This Focus On is about the removal of chlorine, however, many principles in this Focus On can be applied to other oxidants such as chlorine dioxide.