Chlorine Dioxide Measurements – A Technology Review
When discussing the disinfection of water, the use of chlorine (hypochlorite) will usually be one of the first topics discussed, however, did you know that… …Pi provides the technology for the dosing, measurement, and control of an array of other disinfectants including hydrogen peroxide, peracetic acid and chlorine dioxide? Chlorine dioxide, rather than simply being an alternative to chlorine, can be more suitable or effective for many industries and applications. This technology review will examine the properties and uses of chlorine dioxide and the methods that are used to measure its concentration in water. 
 Although, historically, chlorine dioxide has been perceived as hazardous to handle (and in concentrated and gaseous forms, it certainly can be), modern on-site generation technology allows it to be used with more confidence and safety than ever before; as a result, chlorine dioxide has found its place in numerous applications:
Although, historically, chlorine dioxide has been perceived as hazardous to handle (and in concentrated and gaseous forms, it certainly can be), modern on-site generation technology allows it to be used with more confidence and safety than ever before; as a result, chlorine dioxide has found its place in numerous applications: This method relies upon ClO2 oxidizing iodine ions to iodine, which can then be titrated with sodium thiosulphate. Some relatively simple calculations can then be performed, and the concentration of chlorine dioxide can be deduced.
This method relies upon ClO2 oxidizing iodine ions to iodine, which can then be titrated with sodium thiosulphate. Some relatively simple calculations can then be performed, and the concentration of chlorine dioxide can be deduced. This method is based upon the transmission of light. A specific wavelength of light (around 360nm in the case of chlorine dioxide) is passed through a cuvette containing the sample, and the transmission/absorption is measured. The higher the concentration of chlorine dioxide, the lower the level of transmission; this forms the basis of the measurement.
This method is based upon the transmission of light. A specific wavelength of light (around 360nm in the case of chlorine dioxide) is passed through a cuvette containing the sample, and the transmission/absorption is measured. The higher the concentration of chlorine dioxide, the lower the level of transmission; this forms the basis of the measurement. Colorimetric analysis is based on the reaction between ClO2 and a dye, and the measurement of the absorbance of a specific wavelength of light after this reaction takes place. The dyes that can be used include N.N-diethyl-p-phenylenediamine (DPD), chlorophenol red (CPR) and Lissamine Green (LGB). Each of these dyes has its own limitations (e.g. the color of the DPD changes over time, LGB is temperature dependent), but they also have their own positive characteristics.
Colorimetric analysis is based on the reaction between ClO2 and a dye, and the measurement of the absorbance of a specific wavelength of light after this reaction takes place. The dyes that can be used include N.N-diethyl-p-phenylenediamine (DPD), chlorophenol red (CPR) and Lissamine Green (LGB). Each of these dyes has its own limitations (e.g. the color of the DPD changes over time, LGB is temperature dependent), but they also have their own positive characteristics. Designed specifically with online measurement in mind, these electrode-based sensors use a specialized electrolyte held within a membrane cap; this membrane allows ClO2 to diffuse into the electrolyte, where the following takes place at the electrodes:
Designed specifically with online measurement in mind, these electrode-based sensors use a specialized electrolyte held within a membrane cap; this membrane allows ClO2 to diffuse into the electrolyte, where the following takes place at the electrodes: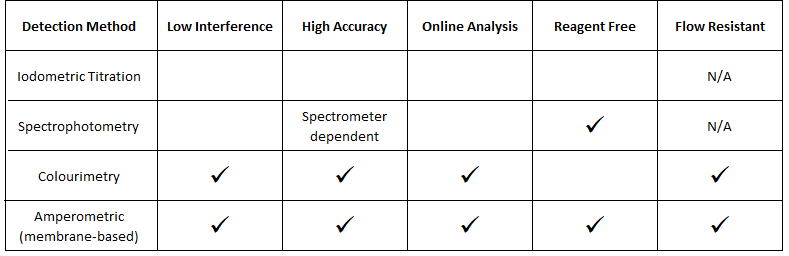 All the approaches above have their own strengths and weaknesses and so the ‘ideal method’ depends upon the need of the end user. As a water instrumentation and control company, Pi sees chlorine dioxide being used most in the kinds of applications that favor continual online analysis, and so the natural choice for Process Instruments is amperometric sensors. Pi’s amperometric DioSense chlorine dioxide analyzers allow for the responsive, real-time measurement of chlorine dioxide without the complex maintenance and reagents needed by online colorimetric systems. Their reagentless operation also means much lower total life costs; a large UK utility company who replaced 330 colorimetric sensors with amperometric probes saw savings of close to a million pounds over a ten year period. Membrane based amperometric probes don’t require the strict flow control required by non-membrane systems, which can be difficult in some applications and industries. Provided some basic conditions are met (such as a lack of surfactants and detergents in the process stream), membrane based sensors are simple to install, calibrate and maintain.
All the approaches above have their own strengths and weaknesses and so the ‘ideal method’ depends upon the need of the end user. As a water instrumentation and control company, Pi sees chlorine dioxide being used most in the kinds of applications that favor continual online analysis, and so the natural choice for Process Instruments is amperometric sensors. Pi’s amperometric DioSense chlorine dioxide analyzers allow for the responsive, real-time measurement of chlorine dioxide without the complex maintenance and reagents needed by online colorimetric systems. Their reagentless operation also means much lower total life costs; a large UK utility company who replaced 330 colorimetric sensors with amperometric probes saw savings of close to a million pounds over a ten year period. Membrane based amperometric probes don’t require the strict flow control required by non-membrane systems, which can be difficult in some applications and industries. Provided some basic conditions are met (such as a lack of surfactants and detergents in the process stream), membrane based sensors are simple to install, calibrate and maintain.

What is chlorine dioxide?
Chlorine dioxide is a compound with the formula ClO2. It appears as a yellowish-green gas and does not hydrolize in water, retaining its structure as a dissolved gas in solution. A powerful oxidizer, it was first suggested as a sterilizing agent as early as 1900 but was only used on a plant scale as late as 19441. The oxidizing properties of chlorine dioxide make it useful for disinfection, and it has several advantages when compared to the more traditional chlorine. For example, it is more effective on specific pathogens such as Legionella2 or Giardia3. When used properly, chlorine dioxide produces less harmful by-products than chlorine, and its disinfection ability is not influenced by pH. Its chemical properties mean that it can be cost effective because lower concentrations are needed compared to other disinfectants.The uses of chlorine dioxide
 Although, historically, chlorine dioxide has been perceived as hazardous to handle (and in concentrated and gaseous forms, it certainly can be), modern on-site generation technology allows it to be used with more confidence and safety than ever before; as a result, chlorine dioxide has found its place in numerous applications:
Although, historically, chlorine dioxide has been perceived as hazardous to handle (and in concentrated and gaseous forms, it certainly can be), modern on-site generation technology allows it to be used with more confidence and safety than ever before; as a result, chlorine dioxide has found its place in numerous applications:- Water disinfection (sewage, industrial processes, cooling towers, foodstuffs etc.)
- Bleaching wood, pulp and paper
- Biofilm removal due to its ability to penetrate the polysaccharide layers of bacterial capsules
- Oxidizing soluble iron and manganese, allowing for easier removal
- Iodometric Titration
- Spectrophotometry
- Colorimetry
- Membrane Amperometry
Iodometric Titration
 This method relies upon ClO2 oxidizing iodine ions to iodine, which can then be titrated with sodium thiosulphate. Some relatively simple calculations can then be performed, and the concentration of chlorine dioxide can be deduced.
This method relies upon ClO2 oxidizing iodine ions to iodine, which can then be titrated with sodium thiosulphate. Some relatively simple calculations can then be performed, and the concentration of chlorine dioxide can be deduced.Advantages:
- Adjusting the pH allows the user to distinguish between Cl2 (chlorine), ClO2 (chlorine dioxide), ClO2⁻ (chlorite) and ClO3⁻ (chlorate). This makes the iodometric method ideal for measuring different chlorine species4
Disadvantages:
- Titrations can be difficult and time consuming to perform, and a higher level of technical skill is required than for other methods
- Chlorine dioxide, chlorite and hypochlorite are not easily distinguished which can cause problems in some applications
- Significant errors can arise if more than one species is present
- Although available as a test kit, the iodometric method has not been adapted for online analysis
Spectrophotometry
 This method is based upon the transmission of light. A specific wavelength of light (around 360nm in the case of chlorine dioxide) is passed through a cuvette containing the sample, and the transmission/absorption is measured. The higher the concentration of chlorine dioxide, the lower the level of transmission; this forms the basis of the measurement.
This method is based upon the transmission of light. A specific wavelength of light (around 360nm in the case of chlorine dioxide) is passed through a cuvette containing the sample, and the transmission/absorption is measured. The higher the concentration of chlorine dioxide, the lower the level of transmission; this forms the basis of the measurement.Advantages:
- This method is relatively free of interferences compared to iodometric titration
- Spectrophotometers are easy to operate, and there is the potential for high accuracy
Disadvantages:
- The overall accuracy is dependent on the photometer used, with higher quality models costing thousands of pounds
- There is a known interference with chlorite, which can introduce significant inaccuracy5
- Online spectrophotometers exist but they aren’t designed with chlorine dioxide in mind
Colorimetric
 Colorimetric analysis is based on the reaction between ClO2 and a dye, and the measurement of the absorbance of a specific wavelength of light after this reaction takes place. The dyes that can be used include N.N-diethyl-p-phenylenediamine (DPD), chlorophenol red (CPR) and Lissamine Green (LGB). Each of these dyes has its own limitations (e.g. the color of the DPD changes over time, LGB is temperature dependent), but they also have their own positive characteristics.
Colorimetric analysis is based on the reaction between ClO2 and a dye, and the measurement of the absorbance of a specific wavelength of light after this reaction takes place. The dyes that can be used include N.N-diethyl-p-phenylenediamine (DPD), chlorophenol red (CPR) and Lissamine Green (LGB). Each of these dyes has its own limitations (e.g. the color of the DPD changes over time, LGB is temperature dependent), but they also have their own positive characteristics.Advantages:
- Colorimetry has higher potential accuracy than spectrophotometry when performed well6. These kinds of tests are well established and are familiar to many technical and site staff
- Online colorimetry products are readily available from a variety of manufacturers
Disadvantages:
- The working range of the test is limited by the concentration of the dye, which places physiochemical constraints on what is possible7
- The purity of the dyes can be a problem, leading to inaccuracies; some studies report the purity of the same dye ranging from 45% to 95%
- Some dyes are not well suited to real time measurements due to longer reaction times
- Online colorimeters require a continual supply of reagents, which can be costly, and blockages in pipes can be quite common
Membrane based amperometric sensors (available from Pi)
 Designed specifically with online measurement in mind, these electrode-based sensors use a specialized electrolyte held within a membrane cap; this membrane allows ClO2 to diffuse into the electrolyte, where the following takes place at the electrodes:
Designed specifically with online measurement in mind, these electrode-based sensors use a specialized electrolyte held within a membrane cap; this membrane allows ClO2 to diffuse into the electrolyte, where the following takes place at the electrodes:- Cathode: ClO2 + 4H⁺ + 5e⁻ → Cl⁻ + 2H2O
- Anode: Cl⁻ + Ag → AgCl + e⁺
Advantages:
- The membrane keeps harmful contaminants away from the electrodes, protecting them
- There is a low dependence on flow rate
- The electrolyte is pre-defined, and so the absence of chlorine dioxide gives zero current; this allows for a one-point calibration method
- No reagents or complex testing methods are required, meaning low operational costs over the lifetime of the sensor
- Continual operation makes these sensors ideal for online measurement
Disadvantages:
- Surfactants and detergents can affect the membrane, allowing water to pass into the electrolyte and cause signal drift
- Water repellent substances such as mineral oils can clog the membrane pores, so chlorine dioxide cannot pass into the electrolyte causing a measurement error
- There is a known ozone interference
How do Process Instruments approach the measurement of chlorine dioxide?
 All the approaches above have their own strengths and weaknesses and so the ‘ideal method’ depends upon the need of the end user. As a water instrumentation and control company, Pi sees chlorine dioxide being used most in the kinds of applications that favor continual online analysis, and so the natural choice for Process Instruments is amperometric sensors. Pi’s amperometric DioSense chlorine dioxide analyzers allow for the responsive, real-time measurement of chlorine dioxide without the complex maintenance and reagents needed by online colorimetric systems. Their reagentless operation also means much lower total life costs; a large UK utility company who replaced 330 colorimetric sensors with amperometric probes saw savings of close to a million pounds over a ten year period. Membrane based amperometric probes don’t require the strict flow control required by non-membrane systems, which can be difficult in some applications and industries. Provided some basic conditions are met (such as a lack of surfactants and detergents in the process stream), membrane based sensors are simple to install, calibrate and maintain.
All the approaches above have their own strengths and weaknesses and so the ‘ideal method’ depends upon the need of the end user. As a water instrumentation and control company, Pi sees chlorine dioxide being used most in the kinds of applications that favor continual online analysis, and so the natural choice for Process Instruments is amperometric sensors. Pi’s amperometric DioSense chlorine dioxide analyzers allow for the responsive, real-time measurement of chlorine dioxide without the complex maintenance and reagents needed by online colorimetric systems. Their reagentless operation also means much lower total life costs; a large UK utility company who replaced 330 colorimetric sensors with amperometric probes saw savings of close to a million pounds over a ten year period. Membrane based amperometric probes don’t require the strict flow control required by non-membrane systems, which can be difficult in some applications and industries. Provided some basic conditions are met (such as a lack of surfactants and detergents in the process stream), membrane based sensors are simple to install, calibrate and maintain.References
- US Environmental Protection Agency: Office of Water, Alternative Disinfectants and Oxidants Manual, chapter 4: Chlorine Dioxide (EPA, 1999)
- Zhang, Z.; McCann, C.; Stout, J.; Piesczynski, S.; Hawks, R.; Vidic, R.; Yu, V. “Safety and Efficacy of Chlorine Dioxide for Legionella control in a Hospital Water System” (2007). Infection Control and Hospital Epidemiology. 28 (8): 1009-1012
- US Environmental Protection Agency: Office of Water, Alternative Disinfectants and Oxidants Manual, chapter 4: Chlorine Dioxide (EPA, 1999)
- Körtvélyesi, Z. Analytical Methods for The Measurement of Chlorine Dioxide and Related Oxychlorine Species in Aqueous Solution (University of Miami, 2004)
- Gordon G. & Körtvélyesi, Z. “Chlorite Ion Interference in The Spectrophotometric Measurement of Chlorine Dioxide” (2004). Journal (American Water Works Association) 96 (9): 81-87
- Min Song et al. “Measurement of Chlorine Dioxide in Water by DPD Colorimetric Method” (2018) IOP Conf. Ser.: Earth Environ. Sci. 108 042102
- Körtvélyesi, Z. Analytical Methods for The Measurement of Chlorine Dioxide and Related Oxychlorine Species in Aqueous Solution (University of Miami, 2004)
Need Help?
Need a Price?
Not in United States?
Share









